ALL THE ANSWERS.
NONE OF THE FLUFF.
Concise, Actionable Medical & Drug Information Resources
Unleash Your Potential & Improve Patient Outcomes
PEPID’s content is specifically designed for you
Please Choose Your Role or Profession
PRACTICING
PROFESSIONAL
HOSPITAL & HEALTH
SYSTEMS
INSTITUTION OR
GROUP
STUDENT OR
RESIDENT
— EST. 1994 —
For over 25 years, PEPID has been a leading developer of clinical and drug information resources and mobile applications for healthcare providers, hospitals, and schools.
PEPID HAS ALL THE TOOLS YOU NEED TO GET THE JOB DONE RIGHT.
MEDICAL CONTENT AND TOOLS
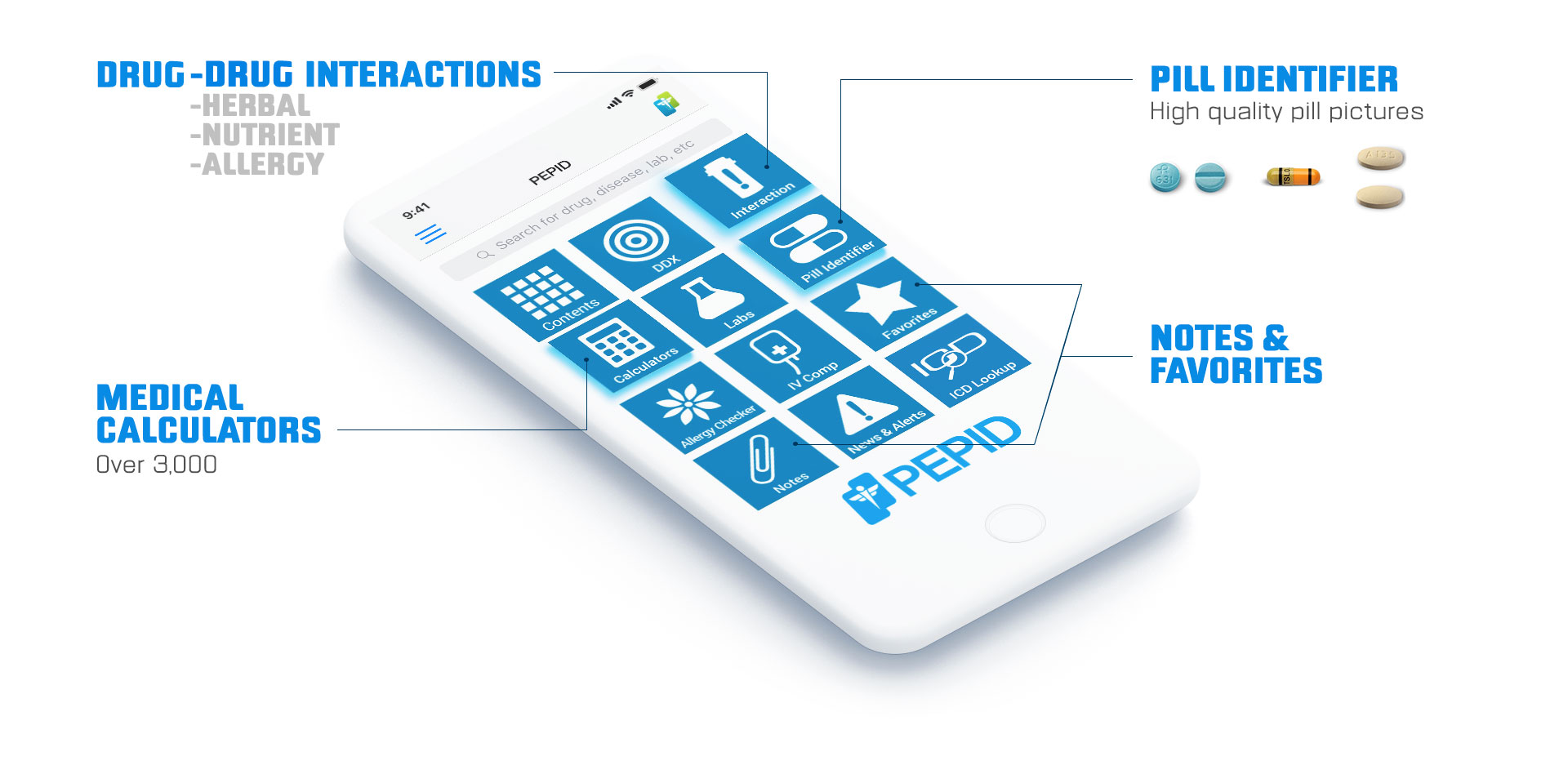
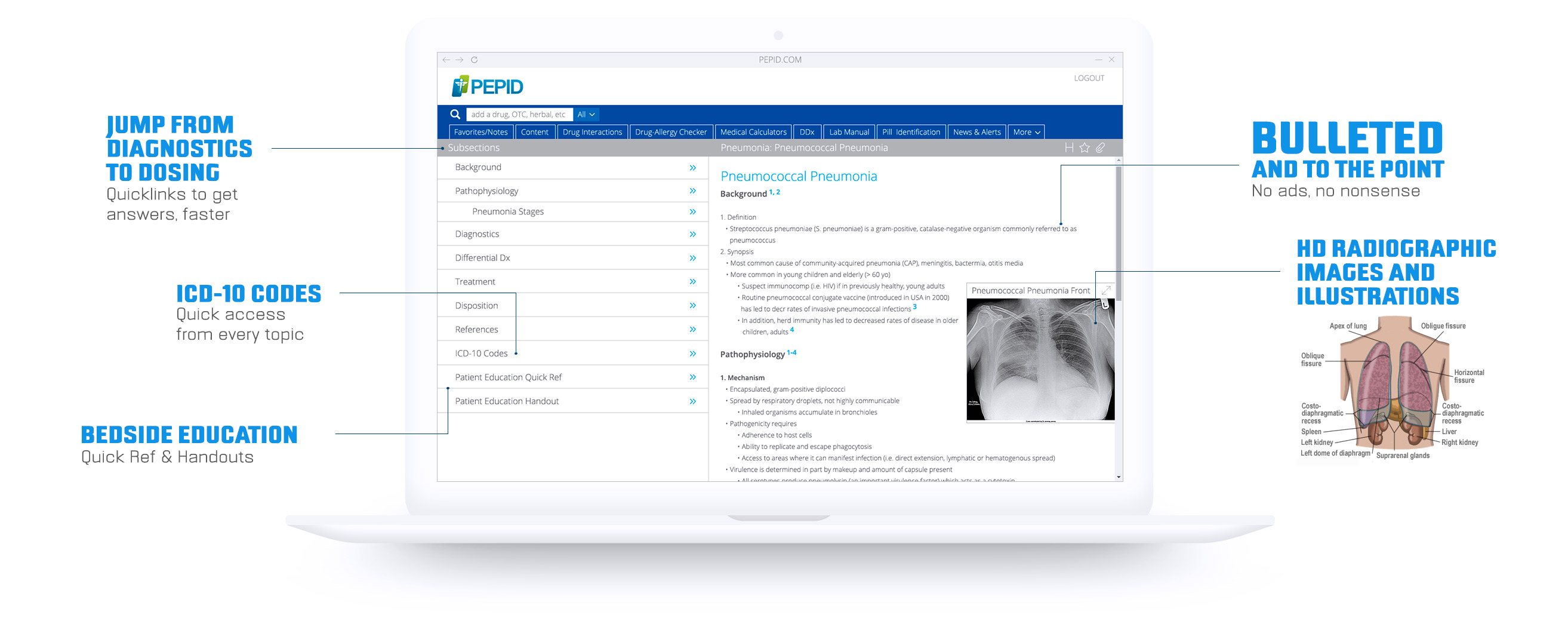
Everything you need in one powerful app
WELCOME TO PEPID.
Established in 1994, PEPID’s complete catalog of healthcare information solutions supports thousands of institutions across the globe. Extensive, intuitive, and easily integrated – PEPID’s clinical and drug databases strengthen critical decision-making at any point-of-care and beyond.WATCH OVERVIEW VIDEO

This is Our Legacy.
As a training physician, Dr. Rosenbloom’s lab coat pockets overflowed with his handwritten, detailed medical and drug reference notes he realized it was invaluable in making diagnostic and therapeutic decisions. When the first personal digital assistants were released, Dr. Rosenbloom copied all of his notes into his handheld computer’s phone book.
To Every Modern Device
Colleagues encouraged Dr. Rosenbloom to expand his electronic notes into a portable, comprehensive medical and drug resource for more widespread use. As a result, in 1994, Dr. Rosenbloom developed the first version of PEPID with an advisory board of more than 35 board-certified physicians and pharmacologists.
Where does PEPID content come from?
Trusted Content Partners. Frequent Updates. Peer-Reviewed.
Ready to get started?
Timely Insights for Healthcare Experts

Omicron Variant: BA.2
Omicron Variant: BA.2 Sublineage By Hannah Morpurgo and Julia Schurr According to the World Health Organization, by February 19, 2022, BA.2 had been detected in 74 countries and 47 US states. BA.2 is a subvariant of Omicron which, according to studies from the UK and...
Read More
EUA: Pfizer-BioNtech Covid-19 Vaccine for Children Ages 5-11
Emergency Use Authorization The CDC now recommends the Pfizer-BioNTech COVID-19 vaccine (BNT162b2) for children ages 5-11 years after the FDA’s EUA approval and support from the Advisory Committee on Immunization Practices (ACIP). The authorization is based on data...
Read More
Omicron Variant – Preliminary Findings
Emergency Use Authorization The CDC now recommends the Pfizer-BioNTech COVID-19 vaccine (BNT162b2) for children ages 5-11 years after the FDA’s EUA approval and support from the Advisory Committee on Immunization Practices (ACIP). The authorization is based on data...
Read More
Peripheral Nerve Blocks for Headaches in the ED
Emergency Use Authorization The CDC now recommends the Pfizer-BioNTech COVID-19 vaccine (BNT162b2) for children ages 5-11 years after the FDA’s EUA approval and support from the Advisory Committee on Immunization Practices (ACIP). The authorization is based on data...
Read More
Not what you were looking for?
or call us 888.321.7828